Life sciences’
trusted cloud software
The most cost-effective drug safety database software for effortless E2B(R3) pharmacovigilance compliance, medical device vigilance, cosmetovigilance and nutrivigilance.
TRUSTED BY THE BEST COMPANIES





MULTIVIGILANCE
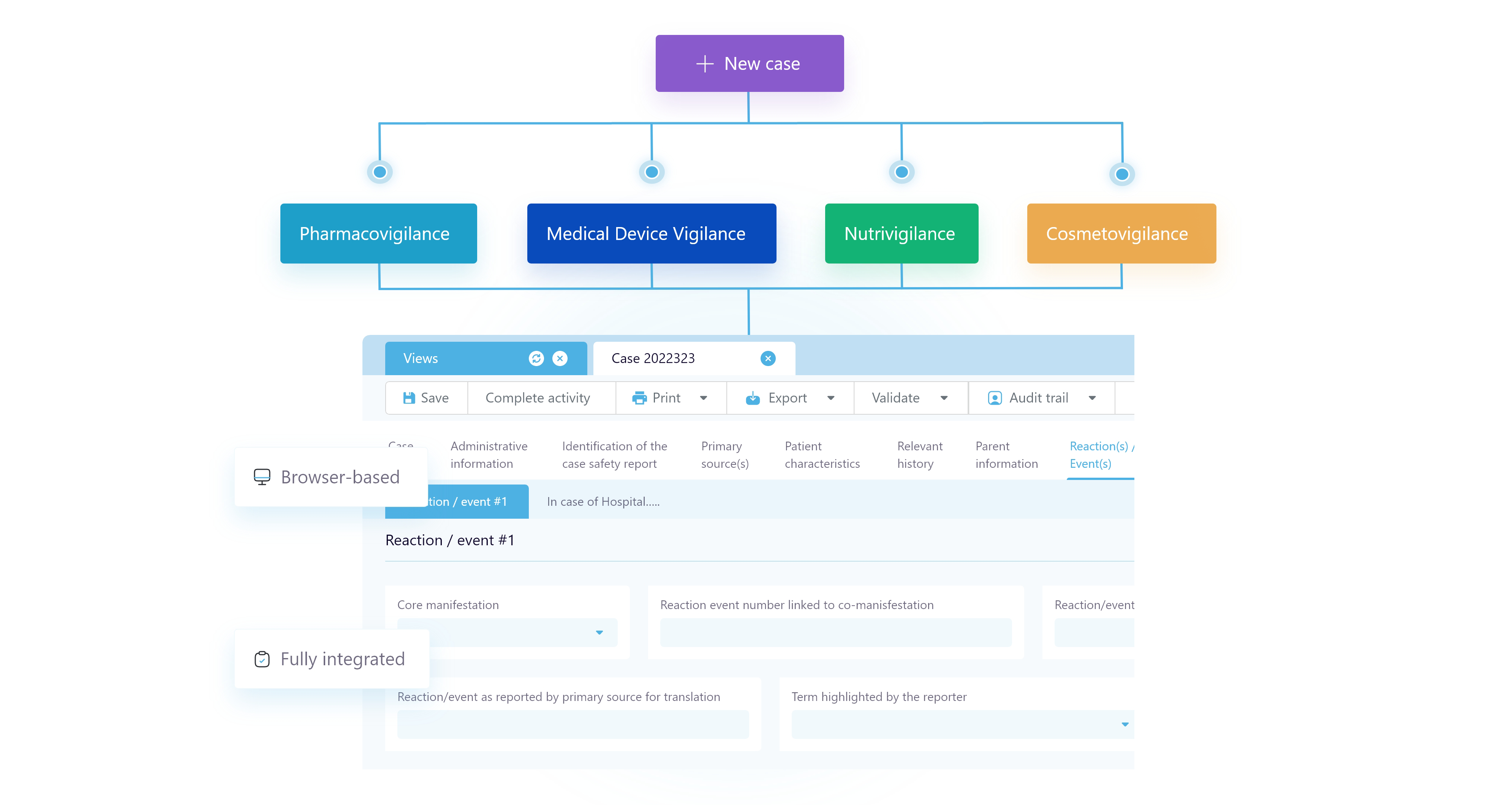
Making multivigilance easy
With SafetyEasy® Suite, you can manage all vigilances on a unified multivigilance management solution. Being a pioneer of SaaS, we accompany you with an agile safety software which adapts to your organisational needs.
The security of your sensitive healthcare and patient safety data is ensured on HDS compliant servers. Consistently compliant with the latest regulations, SafetyEasy® Suite makes meeting drug safety regulatory requirements easier than ever.
FEATURES
Explore SafetyEasy® Suite
AB Cube fulfils vigilance requirements of every scale and scope, from prominent pharmaceuticals to emerging biotechnology firms. We provide drug safety and multivigilance solutions tailored to your needs.
Multivigilance
Harness the power of automation for your global regulatory requirements
Pharmacovigilance
Efficient drug safety operations with the expertise of industry veterans begins here
MedDevice Vigilance
Keep up with the changing regulations while optimising operation efficiency and
Cosmetovigilance
Benefit from the world’s first and only native cosmetics adverse events reporting system
Nutrivigilance
Ensure productive nutrient safety reporting with a native nutrivigilance solution
AB CUBE STATISTICS
Our achievements
We fulfil vigilance requirements of every scale and scope, from research centres to prominent pharmaceutical leaders and emerging biotechnology firms. We provide patient safety and multivigilance solutions tailored to your needs.
16+
Years Expertise
400+
Databases Installed
2K+
SafetyEasy Users
2M+
ICSRs
ABOUT US
Pioneers of SAAS
Industry’s first cloud-based pharmacovigilance software, SafetyEasy® was launched in 2006 when on-premise software was the norm. We accompany academic research centres, CROs and MAHs as their trusted safety software with our reliable technology.
Compliant
Always compliant with the latest regulations, SafetyEasy® Suite conforms to the latest FDA, EMA and MHRA requirements.
Validated
Improved user experience and strengthened security of your healthcare data with a reliable safety system.
Scalable
SafetyEasy® Suite adapts to your vigilance operations from laboratories to global CROs and MAHs.
RECENT ARTICLES
News & events
Meet our experts and engage in a stimulating dialogue, gain some valuable insights from our webinars, grab a coffee at our exhibitor booth, participate in our endeavour of making drug safety easy
AB Cube newsletter: Autumn 2024
A warm welcome to the Autumn edition of our AB Cube newsletter.
In this issue, we lead with the exciting news that Morten Kjær has joined the AB Cube executive team as our new Chief Commercial Officer. As former CEO of BaseCon, Morten brings vast commercial and leadership experience in the PV space, and we’re thrilled to welcome him on board.
New brochure: Next-AI gen Galaxy modules
Our new AB Cube Galaxy brochure is now live.
Discover how your organization can reduce manual processes and enhance efficiency, compliance and signal detection with our next-AI gen Galaxy modules – available as add-ons to our industry-leading SafetyEasy® multivigilance suite.
New brochure: Medical Device Safety Database
Our new Medical Device Safety Database brochure is now online. AB Cube is your complete medical device vigilance partner, trusted by industry leaders to deliver a total safety solution spanning:...



